
Each plant has a purpose. Lets take a look at some of the oldest ones to the new ones.
🌿 Castor Plant – Nature’s High-Performance Oil
The castor plant (Ricinus communis) has been cultivated for over 6,000 years, from ancient Egyptian lamp fuel to modern aerospace lubricants. Its seeds produce a uniquely rich oil—composed of over 90% ricinoleic acid—making it one of the most chemically versatile plant oils on Earth.
At ABOVE, we value castor oil for its:
- Extreme pressure tolerance – ideal for high-stress machinery and racing applications.
- Biodegradability – a sustainable alternative to petroleum lubricants.
- Compatibility – serves as a base for bio-plastics, esters, and performance additives.
Castor is a low-water, high-yield crop that grows well in semi-arid regions, and its oil serves as the foundation for cutting-edge green chemistry and AI-designed lubricants.
From Pharaohs to Formula One—castor oil proves nature’s engineering can go the distance.
Yes — let’s break this down into both the technical composition and the carbon chain structure of castor oil, especially when refined for high-performance or racing applications.
🔬 Technical Composition of Castor Oil (Raw & Refined)
Castor oil is unique among vegetable oils due to its high content of ricinoleic acid, which has functional groups that give it both polar and non-polar characteristics—critical for lubrication.
🔹 Raw Castor Oil Profile:
| Component | Typical Content (%) |
|---|---|
| Ricinoleic acid (C18:1-OH) | 85–90% |
| Oleic acid (C18:1) | 3–5% |
| Linoleic acid (C18:2) | 3–4% |
| Stearic acid (C18:0) | ~1% |
| Dihydroxystearic acid | Trace |
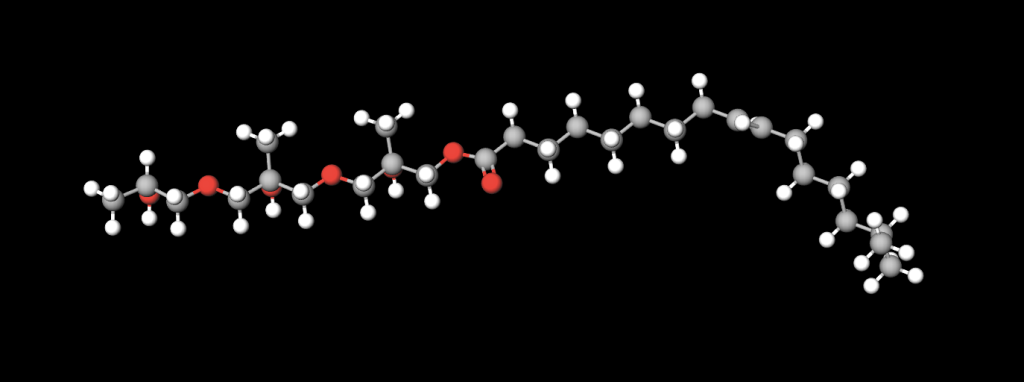
🧪 Ricinoleic Acid Molecular Structure
- C18 backbone, but with:
- One cis double bond at C9=C10
- One hydroxyl (-OH) group at C12
- This structure gives it:
- Hydrogen bonding capability
- High viscosity index
- Exceptional film strength
⚙️ In Racing Applications
When formulated into castor-based racing oil, several processing steps enhance its thermal and oxidative stability:
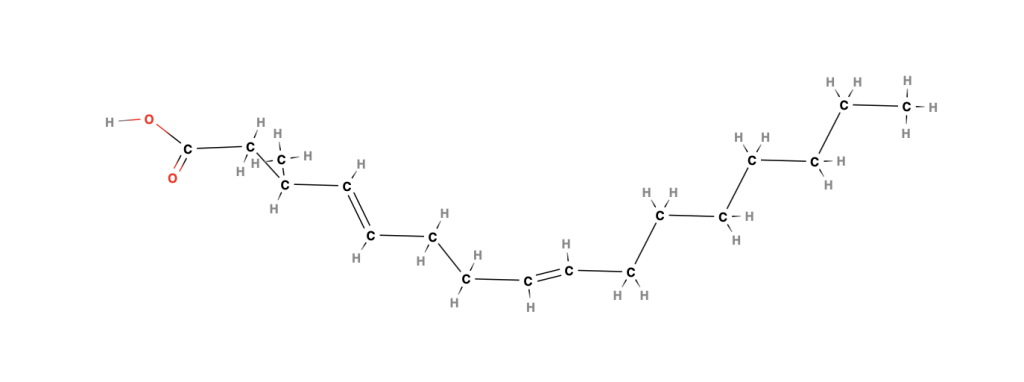
1. Dehydration → Dehydrated Castor Oil (DCO)
- Removes water to create conjugated double bonds
- Improves oxidative stability
2. Esterification (for synthetic esters)
- Forms ricinoleic esters, such as:
- Trimethylolpropane tri-ricinoleate (TMPTE)
- Pentaerythritol tetra-ricinoleate
3. Blending with esters or synthetics (for modern racing blends)
- Sometimes paired with PAO, esters, or alkylated naphthalenes
- Adds cleaner burn, low sludge, and improved cold flow
🧬 Carbon Chain Summary in Castor Racing Oils
| Oil Type | Main Carbon Chain Length | Functional Groups | Stability Use Case |
|---|---|---|---|
| Ricinoleic Acid | C18 | OH at C12, Double bond at C9 | Natural, high film strength |
| Dehydrated Ricinoleic | C18 | Conjugated C=C bonds | Better oxidation resistance |
| Synthetic Ricinoleate Esters | C18 x 3 or x 4 | Multi-branch esters | Used in high-temp racing & aviation oils |
🔥 Why It Works in Racing
- Handles temperatures over 260°C (500°F) without breaking down.
- Builds persistent lubricating films under load.
- Used in 2-stroke racing, rotary engines, and vintage race cars.
- Still favored in some karting and top fuel drag racing blends today.
Leave a Reply